
Francio (Fr)
elemento chimico di numero atomico 87
| Numero Atomico | 87 |
|---|---|
| Massa Atomica | 223 |
| numero di massa | 197 |
| Gruppo | 1 |
|---|---|
| Periodo | 7 |
| Blocco | s |
| protone | 87 p+ |
|---|---|
| neutrone | 110 n0 |
| elettrone | 87 e- |
Proprietà Fisica
| Raggio Atomico | |
|---|---|
| volume molare | |
| Raggio covalente | |
| Metallic Radius | |
| raggio ionico | |
| Crystal Radius | |
| Raggio di van der Waals | |
| massa volumica |
Proprietà Chimica
| energia | |
|---|---|
| Affinità protonica | |
| affinità elettronica | |
| energia di ionizzazione | |
| entalpia di vaporizzazione | |
| entalpia di fusione | |
| entalpia standard di formazione | |
| elettrone | |
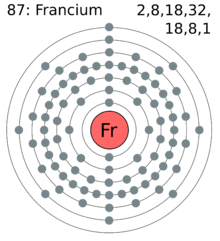
| electron shell | 2, 8, 18, 32, 18, 8, 1 |
| elettrone di valenza | 1 ⓘ |
| configurazione elettronica | [Rn] 7s1ⓘ 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s1 |
| numero di ossidazione | 1 |
| elettronegatività |
0.7
|
| Electrophilicity Index | |
| stato fondamentale della materia | |
| fase della materia | {ERROR}|
| gaseous state of matter | |
| Punto di Ebollizione | |
| Punto di Fusione | |
| pressione critica | |
| Temperatura critica | |
| punto triplo | |
| apparenza | |
| colore | Nero
|
| apparenza | |
| indice di rifrazione | |
| proprietà materiale | |
| Conduttività Termica | |
| dilatazione termica | |
| molar heat capacity | |
| Capacità Termica Specifica | |
| Coefficiente di dilatazione adiabatica | |
| electrical properties | |
| type | |
| conduttività elettrica | |
| resistività elettrica | |
| superconduttività | |
| magnetismo | |
| type | |
| suscettività magnetica (Mass) | |
| suscettività magnetica (Molar) | |
| suscettività magnetica (Volume) | |
| magnetic ordering | |
| punto di Curie | |
| Temperatura di Néel | |
| struttura | |
| Struttura Cristallina | {ERROR} |
| Costante di reticolo | |
| Lattice Angles | |
| proprietà meccanica dei materiali | |
| durezza | |
| Modulo di compressibilità | |
| Modulo di taglio | |
| Young's modulus | |
| modulo di Poisson | |
| velocità del suono | |
| classificazione | |
| Categoria | Attinidi, Alkali metals |
| CAS Group | IA |
| IUPAC Group | IA |
| Glawe Number | 7 |
| Mendeleev Number | 6 |
| Pettifor Number | 7 |
| Geochemical Class | U/Th decay series |
| Classificazione Goldschmidt | synthetic |
other
| Gas Basicity | |
|---|---|
| Polarizzabilità | |
| C6 Dispersion Coefficient | |
| allotrope | |
| cross section | |
| Neutron Mass Absorption | |
| numero quantico | 2S1/2 |
| gruppo spaziale | () |
Isotopi del francio
| Isotopi Stabili | 0 |
|---|---|
| Isotopi Instabili | 37 |
| Natural Isotopes | 0 |
197Fr
| numero di massa | 197 |
|---|---|
| numero neutronico | 110 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 2,3 ± 1,9 ms
|
| spin | 7/2 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 2013 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
198Fr
| numero di massa | 198 |
|---|---|
| numero neutronico | 111 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 15 ± 3 ms
|
| spin | 3 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 2013 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
199Fr
| numero di massa | 199 |
|---|---|
| numero neutronico | 112 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 6,6 ± 2,2 ms
|
| spin | 1/2 |
| nuclear quadrupole moment | 0
|
| data della scoperta o invenzione | 1999 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) |
200Fr
| numero di massa | 200 |
|---|---|
| numero neutronico | 113 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 47,5 ± 2,8 ms
|
| spin | 3 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 1995 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) | |
| β+ SF (β+-delayed fission) |
201Fr
| numero di massa | 201 |
|---|---|
| numero neutronico | 114 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 62,8 ± 1,9 ms
|
| spin | 9/2 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 1980 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) |
202Fr
| numero di massa | 202 |
|---|---|
| numero neutronico | 115 |
| massa atomica relativa | |
| Fattore-g | 1,3 ± 0,016666666666667
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 372 ± 12 ms
|
| spin | 3 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 1980 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) |
203Fr
| numero di massa | 203 |
|---|---|
| numero neutronico | 116 |
| massa atomica relativa | |
| Fattore-g | 0,83111111111111 ± 0,0088888888888889
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 550 ± 10 ms
|
| spin | 9/2 |
| nuclear quadrupole moment | -0,47 ± 0,02
|
| data della scoperta o invenzione | 1967 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) |
204Fr
| numero di massa | 204 |
|---|---|
| numero neutronico | 117 |
| massa atomica relativa | |
| Fattore-g | 1,33 ± 0,01
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 1,75 ± 0,26 s
|
| spin | 3 |
| nuclear quadrupole moment | -0,141 ± 0,009
|
| data della scoperta o invenzione | 1964 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 96% |
| β+ (β+ decay; β+ = ϵ + e+) |
205Fr
| numero di massa | 205 |
|---|---|
| numero neutronico | 118 |
| massa atomica relativa | |
| Fattore-g | 0,84444444444444 ± 0,0066666666666667
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 3,9 ± 0,07 s
|
| spin | 9/2 |
| nuclear quadrupole moment | -0,305 ± 0,018
|
| data della scoperta o invenzione | 1964 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 98.5% |
| β+ (β+ decay; β+ = ϵ + e+) | 1.5% |
206Fr
| numero di massa | 206 |
|---|---|
| numero neutronico | 119 |
| massa atomica relativa | |
| Fattore-g | 1,3233333333333 ± 0,016666666666667
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | |
| spin | 3 |
| nuclear quadrupole moment | -0,354 ± 0,009
|
| data della scoperta o invenzione | 1964 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 88.4% |
| β+ (β+ decay; β+ = ϵ + e+) | 11.6% |
207Fr
| numero di massa | 207 |
|---|---|
| numero neutronico | 120 |
| massa atomica relativa | |
| Fattore-g | 0,86 ± 0,0088888888888889
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 14,8 ± 0,1 s
|
| spin | 9/2 |
| nuclear quadrupole moment | -0,24 ± 0,02
|
| data della scoperta o invenzione | 1964 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 95% |
| β+ (β+ decay; β+ = ϵ + e+) |
208Fr
| numero di massa | 208 |
|---|---|
| numero neutronico | 121 |
| massa atomica relativa | |
| Fattore-g | 0,67285714285714 ± 0,0057142857142857
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 59,1 ± 0,3 s
|
| spin | 7 |
| nuclear quadrupole moment | 0,052 ± 0,011
|
| data della scoperta o invenzione | 1964 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 89% |
| β+ (β+ decay; β+ = ϵ + e+) | 11% |
209Fr
| numero di massa | 209 |
|---|---|
| numero neutronico | 122 |
| massa atomica relativa | |
| Fattore-g | 0,87333333333333 ± 0,011111111111111
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 50,5 ± 0,7 s
|
| spin | 9/2 |
| nuclear quadrupole moment | -0,26 ± 0,02
|
| data della scoperta o invenzione | 1964 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 89% |
| β+ (β+ decay; β+ = ϵ + e+) | 11% |
210Fr
| numero di massa | 210 |
|---|---|
| numero neutronico | 123 |
| massa atomica relativa | |
| Fattore-g | 0,73 ± 0,0083333333333333
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 3,18 ± 0,06 m
|
| spin | 6 |
| nuclear quadrupole moment | 0,21 ± 0,02
|
| data della scoperta o invenzione | 1964 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 71% |
| β+ (β+ decay; β+ = ϵ + e+) |
211Fr
| numero di massa | 211 |
|---|---|
| numero neutronico | 124 |
| massa atomica relativa | |
| Fattore-g | 0,88222222222222 ± 0,011111111111111
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 3,1 ± 0,02 m
|
| spin | 9/2 |
| nuclear quadrupole moment | -0,21 ± 0,02
|
| data della scoperta o invenzione | 1964 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 87% |
| β+ (β+ decay; β+ = ϵ + e+) | 13% |
212Fr
| numero di massa | 212 |
|---|---|
| numero neutronico | 125 |
| massa atomica relativa | |
| Fattore-g | 0,918 ± 0,01
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 20 ± 0,6 m
|
| spin | 5 |
| nuclear quadrupole moment | -0,1 ± 0,002
|
| data della scoperta o invenzione | 1950 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| β+ (β+ decay; β+ = ϵ + e+) | 57% |
| α (α emission) | 43% |
213Fr
| numero di massa | 213 |
|---|---|
| numero neutronico | 126 |
| massa atomica relativa | |
| Fattore-g | 0,88666666666667 ± 0,0022222222222222
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 34,14 ± 0,06 s
|
| spin | 9/2 |
| nuclear quadrupole moment | -0,138 ± 0,003
|
| data della scoperta o invenzione | 1964 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 99.44% |
| β+ (β+ decay; β+ = ϵ + e+) | 0.56% |
214Fr
| numero di massa | 214 |
|---|---|
| numero neutronico | 127 |
| massa atomica relativa | |
| Fattore-g | 0,241 ± 0,016
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 5,51 ± 0,13 ms
|
| spin | 1 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 1967 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
215Fr
| numero di massa | 215 |
|---|---|
| numero neutronico | 128 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 90 ± 4 ns
|
| spin | 9/2 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 1970 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
216Fr
| numero di massa | 216 |
|---|---|
| numero neutronico | 129 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 700 ± 20 ns
|
| spin | 1 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 1970 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) |
217Fr
| numero di massa | 217 |
|---|---|
| numero neutronico | 130 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 22 ± 5 us
|
| spin | 9/2 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 1968 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
218Fr
| numero di massa | 218 |
|---|---|
| numero neutronico | 131 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 1,4 ± 0,5 ms
|
| spin | 1 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 1949 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
219Fr
| numero di massa | 219 |
|---|---|
| numero neutronico | 132 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 22,5 ± 1,7 ms
|
| spin | 9/2 |
| nuclear quadrupole moment | -1,19 ± 0,02
|
| data della scoperta o invenzione | 1948 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
220Fr
| numero di massa | 220 |
|---|---|
| numero neutronico | 133 |
| massa atomica relativa | |
| Fattore-g | -0,66 ± 0,01
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 27,4 ± 0,3 s
|
| spin | 1 |
| nuclear quadrupole moment | 0,487 ± 0,008
|
| data della scoperta o invenzione | 1948 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
| β− (β− decay) | 0.35% |
221Fr
| numero di massa | 221 |
|---|---|
| numero neutronico | 134 |
| massa atomica relativa | |
| Fattore-g | 0,628 ± 0,008
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 4,801 ± 0,005 m
|
| spin | 5/2 |
| nuclear quadrupole moment | -1,02 ± 0,03
|
| data della scoperta o invenzione | 1947 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| α (α emission) | 100% |
| β− (β− decay) | 0.0048% |
| 14C | 8.8% |
222Fr
| numero di massa | 222 |
|---|---|
| numero neutronico | 135 |
| massa atomica relativa | |
| Fattore-g | 0,315 ± 0,005
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 14,2 ± 0,3 m
|
| spin | 2 |
| nuclear quadrupole moment | 0,51 ± 0,04
|
| data della scoperta o invenzione | 1975 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
223Fr
| numero di massa | 223 |
|---|---|
| numero neutronico | 136 |
| massa atomica relativa | |
| Fattore-g | 0,77333333333333 ± 0,0066666666666667
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 22 ± 0,07 m
|
| spin | 3/2 |
| nuclear quadrupole moment | 1,18 ± 0,02
|
| data della scoperta o invenzione | 1939 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
| α (α emission) | 0.006% |
224Fr
| numero di massa | 224 |
|---|---|
| numero neutronico | 137 |
| massa atomica relativa | |
| Fattore-g | 0,4 ± 0,01
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 3,33 ± 0,1 m
|
| spin | 1 |
| nuclear quadrupole moment | 0,523 ± 0,009
|
| data della scoperta o invenzione | 1969 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
225Fr
| numero di massa | 225 |
|---|---|
| numero neutronico | 138 |
| massa atomica relativa | |
| Fattore-g | 0,70666666666667 ± 0,0066666666666667
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 3,95 ± 0,14 m
|
| spin | 3/2 |
| nuclear quadrupole moment | 1,33 ± 0,02
|
| data della scoperta o invenzione | 1969 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
226Fr
| numero di massa | 226 |
|---|---|
| numero neutronico | 139 |
| massa atomica relativa | |
| Fattore-g | 0,0712 ± 0,0014
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 48,5 ± 0,7 s
|
| spin | 1 |
| nuclear quadrupole moment | -1,37 ± 0,03
|
| data della scoperta o invenzione | 1969 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
227Fr
| numero di massa | 227 |
|---|---|
| numero neutronico | 140 |
| massa atomica relativa | |
| Fattore-g | 2,98 ± 0,04
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 2,47 ± 0,03 m
|
| spin | 1/2 |
| nuclear quadrupole moment | 0
|
| data della scoperta o invenzione | 1972 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
228Fr
| numero di massa | 228 |
|---|---|
| numero neutronico | 141 |
| massa atomica relativa | |
| Fattore-g | -0,38 ± 0,005
|
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 38 ± 1 s
|
| spin | 2 |
| nuclear quadrupole moment | 2,41 ± 0,06
|
| data della scoperta o invenzione | 1972 |
| parità | - |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
229Fr
| numero di massa | 229 |
|---|---|
| numero neutronico | 142 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 50,2 ± 0,4 s
|
| spin | 1/2 |
| nuclear quadrupole moment | 0
|
| data della scoperta o invenzione | 1975 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
230Fr
| numero di massa | 230 |
|---|---|
| numero neutronico | 143 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 19,1 ± 0,5 s
|
| spin | 2 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 1987 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
231Fr
| numero di massa | 231 |
|---|---|
| numero neutronico | 144 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 17,6 ± 0,6 s
|
| spin | 1/2 |
| nuclear quadrupole moment | 0
|
| data della scoperta o invenzione | 1985 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
232Fr
| numero di massa | 232 |
|---|---|
| numero neutronico | 145 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 5,5 ± 0,6 s
|
| spin | 5 |
| nuclear quadrupole moment | |
| data della scoperta o invenzione | 1990 |
| parità |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
| β− SF (β−-delayed fission) |
233Fr
| numero di massa | 233 |
|---|---|
| numero neutronico | 146 |
| massa atomica relativa | |
| Fattore-g | |
| natural abundance | |
| radioattività | ☢️ radioactive element |
| emivita | 900 ± 100 ms
|
| spin | 1/2 |
| nuclear quadrupole moment | 0
|
| data della scoperta o invenzione | 2010 |
| parità | + |
| tipo di decadimento | intensità |
|---|---|
| β− (β− decay) | 100% |
| β− n (β−-delayed neutron emission) |
storia
| scoperto o inventato da | Marguerite Derey |
|---|---|
| luogo di scoperta | France |
| data della scoperta o invenzione | 1939 |
| etimologia | Named for France, the nation of its discovery. |
| pronuncia | FRAN-si-em (inglese) |
sorgente
| Abbondanza | |
|---|---|
| Abbondanza sulla crosta terrestre | |
| natural abundance (oceano) | |
| natural abundance (corpo umano) | 0 %
|
| natural abundance (meteoroide) | |
| natural abundance (Sole) | |
| Abbondanza nell'universo | 0 %
|




