
Francium (Fr)
| Atomic Number | 87 |
|---|---|
| Atomic Weight | 223 |
| Mass Number | 197 |
| Group | 1 |
|---|---|
| Period | 7 |
| Block | s |
| Protons | 87 p+ |
|---|---|
| Neutrons | 110 n0 |
| Electrons | 87 e- |
Physical Properties
| Atomic Radius | |
|---|---|
| Molar Volume | |
| Covalent Radius | |
| Metallic Radius | |
| Ionic Radius | |
| Crystal Radius | |
| Van der Waals Radius | |
| Density |
Chemical Properties
| Energy | |
|---|---|
| Proton Affinity | |
| Electron Affinity | |
| Ionization Energy | |
| Heat of Vaporization | |
| Heat of Fusion | |
| Heat of Formation | |
| Electrons | |
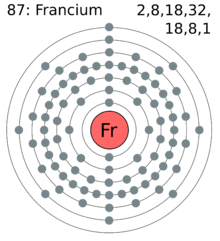
| Electron Shells | 2, 8, 18, 32, 18, 8, 1 |
| Valence Electrons | 1 ⓘ |
| Electron Configuration | [Rn] 7s1ⓘ 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s1 |
| Oxidation States | 1 |
| Electronegativity |
0.7
|
| Electrophilicity Index | |
| States of Matter | |
| Phase | {ERROR}|
| Gas Phase | |
| Boiling Point | |
| Melting Point | |
| Critical Pressure | |
| Critical Temperature | |
| Triple Point | |
| Visual | |
Francium is a Black. Fr has a CPK of #ffffff, Jmol of #420066, and MOLCAS GV of #420066. | |
| Color | Black
|
| Appearance | |
| Refractive Index | |
| Thermodynamic Properties | |
| Thermal Conductivity | |
| Thermal Expansion | |
| Molar Heat Capacity | |
| Specific Heat Capacity | |
| Heat Capacity Ratio (Adiabatic Index) | |
| Electrical Properties | |
| Type | |
| Electrical Conductivity | |
| Electrical Resistivity | |
| Superconducting Point | |
| Magnetism | |
| Type | |
| Magnetic Susceptibility (Mass) | |
| Magnetic Susceptibility (Molar) | |
| Magnetic Susceptibility (Volume) | |
| Magnetic Ordering | |
| Curie Point | |
| Neel Point | |
| Structure | |
The Crystal Structure of Francium is BCC. The lattice angles of Fr is . | |
| Crystal Structure | {ERROR} |
| Lattice Constant | |
| Lattice Angles | |
| Mechanical Properties | |
| Hardness | |
| Bulk Modulus | |
| Shear Modulus | |
| Young's Modulus | |
| Poisson Ratio | |
| Speed of Sound | |
| Classification | |
The CAS Group of Francium is IA. The IUPAC Group of Fr is IA. The Glawe Number of Element 87 is 7. The Mendeleev Number of Francium (Fr) is 6. The Pettifor Number of Francium is 7. The Geochemical Class of Fr is U/Th decay series. The Goldschmidt Class of Element 87 is synthetic. | |
| Category | Actinides, Alkali metals |
| CAS Group | IA |
| IUPAC Group | IA |
| Glawe Number | 7 |
| Mendeleev Number | 6 |
| Pettifor Number | 7 |
| Geochemical Class | U/Th decay series |
| Goldschmidt Class | synthetic |
Other
The Dipole Polarizability of Francium is 317.8 plus or minus 2.4 a₀. The Allotropes of Fr is . The Quantum Numbers of Element 87 is 2S1/2. The Space Group of Francium (Fr) is ().
| Gas Basicity | |
|---|---|
| Dipole Polarizability | |
| C6 Dispersion Coefficient | |
| Allotropes | |
| Neutron Cross Section | |
| Neutron Mass Absorption | |
| Quantum Numbers | 2S1/2 |
| Space Group | () |
Isotopes of Francium
| Stable Isotopes | 0 |
|---|---|
| Unstable Isotopes | 37 |
| Natural Isotopes | 0 |
197Fr
| Mass Number | 197 |
|---|---|
| Neutron Number | 110 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 2.3 ± 1.9 ms
|
| Spin | 7/2 |
| Quadrupole Moment | |
| Discovery Year | 2013 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
198Fr
| Mass Number | 198 |
|---|---|
| Neutron Number | 111 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 15 ± 3 ms
|
| Spin | 3 |
| Quadrupole Moment | |
| Discovery Year | 2013 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
199Fr
| Mass Number | 199 |
|---|---|
| Neutron Number | 112 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 6.6 ± 2.2 ms
|
| Spin | 1/2 |
| Quadrupole Moment | 0
|
| Discovery Year | 1999 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) |
200Fr
| Mass Number | 200 |
|---|---|
| Neutron Number | 113 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 47.5 ± 2.8 ms
|
| Spin | 3 |
| Quadrupole Moment | |
| Discovery Year | 1995 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) | |
| β+ SF (β+-delayed fission) |
201Fr
| Mass Number | 201 |
|---|---|
| Neutron Number | 114 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 62.8 ± 1.9 ms
|
| Spin | 9/2 |
| Quadrupole Moment | |
| Discovery Year | 1980 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) |
202Fr
| Mass Number | 202 |
|---|---|
| Neutron Number | 115 |
| Relative Atomic Mass | |
| G-Factor | 1.3 ± 0.016666666666667
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 372 ± 12 ms
|
| Spin | 3 |
| Quadrupole Moment | |
| Discovery Year | 1980 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) |
203Fr
| Mass Number | 203 |
|---|---|
| Neutron Number | 116 |
| Relative Atomic Mass | |
| G-Factor | 0.83111111111111 ± 0.0088888888888889
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 550 ± 10 ms
|
| Spin | 9/2 |
| Quadrupole Moment | -0.47 ± 0.02
|
| Discovery Year | 1967 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) |
204Fr
| Mass Number | 204 |
|---|---|
| Neutron Number | 117 |
| Relative Atomic Mass | |
| G-Factor | 1.33 ± 0.01
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 1.75 ± 0.26 s
|
| Spin | 3 |
| Quadrupole Moment | -0.141 ± 0.009
|
| Discovery Year | 1964 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 96% |
| β+ (β+ decay; β+ = ϵ + e+) |
205Fr
| Mass Number | 205 |
|---|---|
| Neutron Number | 118 |
| Relative Atomic Mass | |
| G-Factor | 0.84444444444444 ± 0.0066666666666667
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 3.9 ± 0.07 s
|
| Spin | 9/2 |
| Quadrupole Moment | -0.305 ± 0.018
|
| Discovery Year | 1964 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 98.5% |
| β+ (β+ decay; β+ = ϵ + e+) | 1.5% |
206Fr
| Mass Number | 206 |
|---|---|
| Neutron Number | 119 |
| Relative Atomic Mass | |
| G-Factor | 1.3233333333333 ± 0.016666666666667
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | |
| Spin | 3 |
| Quadrupole Moment | -0.354 ± 0.009
|
| Discovery Year | 1964 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 88.4% |
| β+ (β+ decay; β+ = ϵ + e+) | 11.6% |
207Fr
| Mass Number | 207 |
|---|---|
| Neutron Number | 120 |
| Relative Atomic Mass | |
| G-Factor | 0.86 ± 0.0088888888888889
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 14.8 ± 0.1 s
|
| Spin | 9/2 |
| Quadrupole Moment | -0.24 ± 0.02
|
| Discovery Year | 1964 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 95% |
| β+ (β+ decay; β+ = ϵ + e+) |
208Fr
| Mass Number | 208 |
|---|---|
| Neutron Number | 121 |
| Relative Atomic Mass | |
| G-Factor | 0.67285714285714 ± 0.0057142857142857
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 59.1 ± 0.3 s
|
| Spin | 7 |
| Quadrupole Moment | 0.052 ± 0.011
|
| Discovery Year | 1964 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 89% |
| β+ (β+ decay; β+ = ϵ + e+) | 11% |
209Fr
| Mass Number | 209 |
|---|---|
| Neutron Number | 122 |
| Relative Atomic Mass | |
| G-Factor | 0.87333333333333 ± 0.011111111111111
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 50.5 ± 0.7 s
|
| Spin | 9/2 |
| Quadrupole Moment | -0.26 ± 0.02
|
| Discovery Year | 1964 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 89% |
| β+ (β+ decay; β+ = ϵ + e+) | 11% |
210Fr
| Mass Number | 210 |
|---|---|
| Neutron Number | 123 |
| Relative Atomic Mass | |
| G-Factor | 0.73 ± 0.0083333333333333
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 3.18 ± 0.06 m
|
| Spin | 6 |
| Quadrupole Moment | 0.21 ± 0.02
|
| Discovery Year | 1964 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 71% |
| β+ (β+ decay; β+ = ϵ + e+) |
211Fr
| Mass Number | 211 |
|---|---|
| Neutron Number | 124 |
| Relative Atomic Mass | |
| G-Factor | 0.88222222222222 ± 0.011111111111111
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 3.1 ± 0.02 m
|
| Spin | 9/2 |
| Quadrupole Moment | -0.21 ± 0.02
|
| Discovery Year | 1964 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 87% |
| β+ (β+ decay; β+ = ϵ + e+) | 13% |
212Fr
| Mass Number | 212 |
|---|---|
| Neutron Number | 125 |
| Relative Atomic Mass | |
| G-Factor | 0.918 ± 0.01
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 20 ± 0.6 m
|
| Spin | 5 |
| Quadrupole Moment | -0.1 ± 0.002
|
| Discovery Year | 1950 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| β+ (β+ decay; β+ = ϵ + e+) | 57% |
| α (α emission) | 43% |
213Fr
| Mass Number | 213 |
|---|---|
| Neutron Number | 126 |
| Relative Atomic Mass | |
| G-Factor | 0.88666666666667 ± 0.0022222222222222
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 34.14 ± 0.06 s
|
| Spin | 9/2 |
| Quadrupole Moment | -0.138 ± 0.003
|
| Discovery Year | 1964 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 99.44% |
| β+ (β+ decay; β+ = ϵ + e+) | 0.56% |
214Fr
| Mass Number | 214 |
|---|---|
| Neutron Number | 127 |
| Relative Atomic Mass | |
| G-Factor | 0.241 ± 0.016
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 5.51 ± 0.13 ms
|
| Spin | 1 |
| Quadrupole Moment | |
| Discovery Year | 1967 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
215Fr
| Mass Number | 215 |
|---|---|
| Neutron Number | 128 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 90 ± 4 ns
|
| Spin | 9/2 |
| Quadrupole Moment | |
| Discovery Year | 1970 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
216Fr
| Mass Number | 216 |
|---|---|
| Neutron Number | 129 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 700 ± 20 ns
|
| Spin | 1 |
| Quadrupole Moment | |
| Discovery Year | 1970 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
| β+ (β+ decay; β+ = ϵ + e+) |
217Fr
| Mass Number | 217 |
|---|---|
| Neutron Number | 130 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 22 ± 5 us
|
| Spin | 9/2 |
| Quadrupole Moment | |
| Discovery Year | 1968 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
218Fr
| Mass Number | 218 |
|---|---|
| Neutron Number | 131 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 1.4 ± 0.5 ms
|
| Spin | 1 |
| Quadrupole Moment | |
| Discovery Year | 1949 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
219Fr
| Mass Number | 219 |
|---|---|
| Neutron Number | 132 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 22.5 ± 1.7 ms
|
| Spin | 9/2 |
| Quadrupole Moment | -1.19 ± 0.02
|
| Discovery Year | 1948 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
220Fr
| Mass Number | 220 |
|---|---|
| Neutron Number | 133 |
| Relative Atomic Mass | |
| G-Factor | -0.66 ± 0.01
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 27.4 ± 0.3 s
|
| Spin | 1 |
| Quadrupole Moment | 0.487 ± 0.008
|
| Discovery Year | 1948 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
| β− (β− decay) | 0.35% |
221Fr
| Mass Number | 221 |
|---|---|
| Neutron Number | 134 |
| Relative Atomic Mass | |
| G-Factor | 0.628 ± 0.008
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 4.801 ± 0.005 m
|
| Spin | 5/2 |
| Quadrupole Moment | -1.02 ± 0.03
|
| Discovery Year | 1947 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| α (α emission) | 100% |
| β− (β− decay) | 0.0048% |
| 14C | 8.8% |
222Fr
| Mass Number | 222 |
|---|---|
| Neutron Number | 135 |
| Relative Atomic Mass | |
| G-Factor | 0.315 ± 0.005
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 14.2 ± 0.3 m
|
| Spin | 2 |
| Quadrupole Moment | 0.51 ± 0.04
|
| Discovery Year | 1975 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
223Fr
| Mass Number | 223 |
|---|---|
| Neutron Number | 136 |
| Relative Atomic Mass | |
| G-Factor | 0.77333333333333 ± 0.0066666666666667
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 22 ± 0.07 m
|
| Spin | 3/2 |
| Quadrupole Moment | 1.18 ± 0.02
|
| Discovery Year | 1939 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
| α (α emission) | 0.006% |
224Fr
| Mass Number | 224 |
|---|---|
| Neutron Number | 137 |
| Relative Atomic Mass | |
| G-Factor | 0.4 ± 0.01
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 3.33 ± 0.1 m
|
| Spin | 1 |
| Quadrupole Moment | 0.523 ± 0.009
|
| Discovery Year | 1969 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
225Fr
| Mass Number | 225 |
|---|---|
| Neutron Number | 138 |
| Relative Atomic Mass | |
| G-Factor | 0.70666666666667 ± 0.0066666666666667
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 3.95 ± 0.14 m
|
| Spin | 3/2 |
| Quadrupole Moment | 1.33 ± 0.02
|
| Discovery Year | 1969 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
226Fr
| Mass Number | 226 |
|---|---|
| Neutron Number | 139 |
| Relative Atomic Mass | |
| G-Factor | 0.0712 ± 0.0014
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 48.5 ± 0.7 s
|
| Spin | 1 |
| Quadrupole Moment | -1.37 ± 0.03
|
| Discovery Year | 1969 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
227Fr
| Mass Number | 227 |
|---|---|
| Neutron Number | 140 |
| Relative Atomic Mass | |
| G-Factor | 2.98 ± 0.04
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 2.47 ± 0.03 m
|
| Spin | 1/2 |
| Quadrupole Moment | 0
|
| Discovery Year | 1972 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
228Fr
| Mass Number | 228 |
|---|---|
| Neutron Number | 141 |
| Relative Atomic Mass | |
| G-Factor | -0.38 ± 0.005
|
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 38 ± 1 s
|
| Spin | 2 |
| Quadrupole Moment | 2.41 ± 0.06
|
| Discovery Year | 1972 |
| Parity | - |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
229Fr
| Mass Number | 229 |
|---|---|
| Neutron Number | 142 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 50.2 ± 0.4 s
|
| Spin | 1/2 |
| Quadrupole Moment | 0
|
| Discovery Year | 1975 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
230Fr
| Mass Number | 230 |
|---|---|
| Neutron Number | 143 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 19.1 ± 0.5 s
|
| Spin | 2 |
| Quadrupole Moment | |
| Discovery Year | 1987 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
231Fr
| Mass Number | 231 |
|---|---|
| Neutron Number | 144 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 17.6 ± 0.6 s
|
| Spin | 1/2 |
| Quadrupole Moment | 0
|
| Discovery Year | 1985 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
232Fr
| Mass Number | 232 |
|---|---|
| Neutron Number | 145 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 5.5 ± 0.6 s
|
| Spin | 5 |
| Quadrupole Moment | |
| Discovery Year | 1990 |
| Parity |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
| β− SF (β−-delayed fission) |
233Fr
| Mass Number | 233 |
|---|---|
| Neutron Number | 146 |
| Relative Atomic Mass | |
| G-Factor | |
| Abundance | |
| Radioactivity | ☢️ Radioactive |
| Half Life | 900 ± 100 ms
|
| Spin | 1/2 |
| Quadrupole Moment | 0
|
| Discovery Year | 2010 |
| Parity | + |
| Decay Mode | Intensity |
|---|---|
| β− (β− decay) | 100% |
| β− n (β−-delayed neutron emission) |
History
Francium was discovered in 1939 by Marguerite Perey of the Curie Institute in Paris, France. It was discovered when she was researching the radioactive decay of actinium-227. Marguerite Perey discovered that francium-223 is made naturally when actinium-227 emits an alpha-particle. Francium was named after France
| Discoverers | Marguerite Derey |
|---|---|
| Discovery Location | France |
| Discovery Year | 1939 |
| Etymology (Name Origin) | Named for France, the nation of its discovery. |
| Pronunciation | FRAN-si-em (English) |
Uses
Sources
Formed by decay of actinium. Chemical properties similar to cesium. Decays to radium or astatine.
| Abundance | |
|---|---|
| Abundance in Earth's crust | |
| Natural Abundance (Oceans) | |
| Natural Abundance (Human Body) | 0 %
|
| Natural Abundance (Meteor) | |
| Natural Abundance (Sun) | |
| Abundance in Universe | 0 %
|
Nuclear Screening Constants
Also Known As
- Fr
- element 87
- 87Fr
- catium
- eka-cesium
- eka-caesium
Translations
- Frenchfrancium
- Norwegian Bokmålfrancium
- Russianфранций
- Spanishfrancio
- GermanFrancium
- Italianfrancio
- Norwegian Nynorskfrancium
- Hungarianfrancium
- Amharicፍራንሺየም
- AragoneseFrancio
- Arabicفرانسيوم
- AzerbaijaniFransium
- Belarusianфранцый
- Bulgarianфранций
- Banglaফ্র্যান্সিয়াম
- BretonFrankiom
- Bosnianfrancij
- Catalanfranci
- CorsicanFranciu
- Czechfrancium
- ChuvashФранци
- WelshFfransiwm
- Danishfrancium
- Greekφράγκιο
- Esperantofranciumo
- Estonianfrantsium
- Basquefrantzio
- Persianفرانسیم
- Finnishfrankium
- FriulianFranci
- IrishFrainciam
- GalicianFrancio
- GuaraniHyãsiakuarepoti
- ManxFrankium
- Hakka Chinesefrancium
- Hebrewפרנציום
- Fiji HindiFrancium
- Croatianfrancij
- Haitian CreoleFransyòm
- Armenianֆրանսիում
- Interlinguafrancium
- Indonesianfransium
- Idofrancio
- Icelandicfransín
- Japaneseフランシウム
- Lojbanfasysodna
- JavaneseFransium
- Georgianფრანციუმი
- KazakhФранций
- Kannadaಫ್ರಾನ್ಸಿಯಮ್
- Korean프랑슘
- KurdishFransiyûm
- KomiФранций
- Latinfrancium
- LuxembourgishFrancium
- LigurianFransio
- Lithuanianfrancis
- Latvianfrancijs
- Malayalamഫ്രാൻസിയം
- Marathiफ्रान्सियम
- Western MariФранций
- MalayFransium
- Burmeseဖရန်စီယမ်
- Mazanderaniفرانسیوم
- Dutchfrancium
- Polishfrans
- Western Panjabiفرانسیم
- Portuguesefrâncio
- QuechuaFransyu
- Romanianfranciu
- YakutФрансиум
- Sicilianfranciu
- Serbo-CroatianFrancijum
- Slovakfrancium
- Slovenianfrancij
- AlbanianFranciumi
- Serbianфранцијум
- Saterland FrisianFrancium
- Swedishfrancium
- SwahiliFransi
- Tamilபிரான்சீயம்
- Thaiแฟรนเซียม
- Turkishfransiyum
- Uyghurفرانسىي
- Ukrainianфранцій
- Urduفرانسیئم
- VepsFrancii
- Vietnamesefranxi
- WalloonFranciom
- WarayFransyo
- KalmykФранциум
- YorubaFránsíọ̀m
- Chinese钫
- CebuanoPransyo
- Central Kurdishفرانسیۆم
- Macedonianфранциум
- TagalogPransyo
- Punjabiਫ਼ਰਾਂਸੀਅਮ
- Odiaଫ୍ରାନ୍ସିଅମ
- Gujaratiફ્રાંસીયમ
- German (Switzerland)Francium
- English (Canada)Francium
- English (United Kingdom)francium
- Portuguese (Brazil)frâncio
- Cantonese鈁
- OccitanFranci
- Chinese (Simplified)钫
- TatarФранций
- PiedmonteseFransio
- Newariफ्रान्सियम
- SomaliFaransiyaam
- Belarusian (Taraskievica orthography)франц
- Chinese (Taiwan)鍅
- Chinese (Hong Kong SAR China)鈁
- Chinese (Traditional)鍅
- UzbekFransiy
- Sanskritफ्रान्सियम
- KyrgyzФранций
- LimburgishFrancium
- FaroeseFransium
- Teluguఫ్రాన్సియం
- Hindiक्षुद्रातु
- Maltesefranċju
- Nepaliफ्रान्सियम
- LombardFrancio
- Paliफ्रान्सियम
- Gan Chinese鈁
- Mongolianфранци
- cdoFrancium
- Min Nan ChineseFrancium
- AsturianFranciu
- Bhojpuriफ्रंशियम
- Tibetanཧྦ་རན་སིམ།
- Scottish GaelicFraingium
- Lingua Franca Novafransio
- AfrikaansFrankium
- kbpFransɩyɔm
- Literary Chinese鍅
- Sinhalaෆ්රැන්සියම්
- Northern FrisianFrantsium
- oloFrancii (aineh)
- Volapükfransin
- AromanianFranciu
- Wu Chinese钫
- TajikФранcий
- Low GermanFrancium
- SardinianFràntziu
- Moroccan Arabicفرانسيوم
- Pashtoفرانسيوم
- Egyptian Arabicفرانسيوم
- BikolPransyo
- CornishFrankiom
- Manipuriꯐ꯭ꯔꯥꯟꯁꯤꯌꯝ
- BalineseFransium
- ZuluIShabalalambi




